First Pharmacy Conference at Baycrest Centre for Geriatric Care
Christine Oyugi, BSc, Managing Editor, Geriatrics & Aging.
Speakers
- Osteoporosis Update:
Tom Brown, PharmD, Pharmacist, Women's Health Pharmacy Department, Sunnybrook & Women's College Health Sciences Centre, Toronto, ON. - Diabetes and the Elderly:
Dr. Christine Papoushek, PharmD, University Health Network, Department of Family and Community Medicine/Pharmacy, Toronto, ON. - Pharmacological Management of Congestive Heart Failure:
Fran L. Paradiso-Hardy, BScPhm, MSc Pharm, Clinical Co-ordinator/Infectious Diseases, Department of Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON. - New Evidence and Guidelines in Osteoarthritis:
Natalie Kennie, PharmD, Primary Care Pharmacist, St. Michael's Hospital Health Centre, Toronto, ON.
Elderly patients are at high risk for medication-related problems due to age-related physiological changes, a higher incidence of comorbid illnesses and greater use of both prescription and over-the-counter medications. As a result, older adults are at increased risk of developing adverse drug events. It is important for physicians to regularly review the drug regimen of any older patient, in order to determine if the drug is effective, monitor for adverse drug events and recommend newer alternative therapies, as they become available. These points were addressed at the 'First Pharmacy Conference on Medication Use in the Geriatric Population' held at the Baycrest Centre for Geriatric Care. This article summarizes some of the major points addressed at this conference.
I. Osteoporosis Update
The objectives of this talk were to illustrate the role of combination therapy in the management of osteoporosis; discuss the value of guidelines in managing osteoporosis; and demonstrate the application of new evidence to managing complex patients. Dr. Brown presented four case studies by way of achieving these objectives.
Case Synopses
Case One
A 57-year-old Caucasian female who has been on Hormone Replacement Therapy (HRT) for the past four years for vasomotor symptoms. She was diagnosed with osteopenia two years ago and takes conjugated equine estrogen (CEE) 0.625mg, and Medroxyprogesterone acetate 2.5mg daily. She has a familial history of osteoporosis, myocardial infarct (MI) and breast cancer, and smokes a pack of cigarettes a day. Her BMD was -2.3 spine and -1.8 hip in January 2000, -2.6 spine and -2.2 hip in January 2002.
Should we add a bisphosphonate to the HRT regimen? Several studies indicate beneficial effects of adding a bisphosphonate to ongoing HRT in postmenopausal women. Patients on HRT and etidronate have a significantly greater BMD when compared to women on monotherapy. A study of 428 postmenopausal women with osteoporosis, who had been receiving HRT for at least one year, demonstrated that adding a bisphosphonate (alendronate) significantly increased bone mass at both spine and hip trochanter.1 Therefore, patients who have failed both HRT and bisphosphonate monotherapy should be considered for combination therapy. Although BMD improves, several sub-group analyses demonstrate that changes in BMD cannot fully account for anti-fracture efficacy.
Case Two
A 70-year-old Asian women with osteoporosis. Her mother fractured a hip at the age of 86 years and her father developed colon cancer at 64 years. Her BMD is spine -2.8 and hip -1.9. Her total cholesterol is 6.85 mmol/L, LDL 4.97mmol/L, HDL 1.32 mmol/L and triglyceride 1.84mmol/L.
Currently, several guidelines exist for the treatment of osteoporosis. Unfortunately, the guidelines have varying recommendations, are often outdated and tend to focus on risk factors and initiating therapy, without proper advice on monitoring or follow-up. The guidelines from several organizations are listed below:
- The National Osteoporosis Foundation (NOF)--First consider HRT and then Alendronate in patients who are unwilling or unable to take HRT, or who fail on HRT. If both bisphosphonates and HRT fail, then consider calcitonin. Raloxifene, a selective estrogen-receptor modulator (SERM), is given as an alternative therapy.
- The American Association of Clinical Endocrinologists (AACE)--First priority is given to FDA-approved medications for prevention and treatment. There is level 1 evidence that bisphosphonates, calcitonin and raloxifene decrease vertebral hip fractures.
- The Ontario Program for Optimal Therapeutics (OPOT)--Try estrogen, bisphosphonate or SERM. If unable to take any of the three, or if immobilized and with acute fracture, try calcitonin.
- The Osteoporosis Society of Canada's guidelines, which are in the process of being developed, suggests the use of bisphosphonates and raloxifene. For this case, Dr. Brown suggests the use of raloxifene.
Case Three
A 65-year-old Caucasian male with osteoporosis indicated by a BMD of -2.4 spine and -2.6 hip. His medications are actenolol, enalapril, atorvastatin and ASA. He had an MI at 45 and stopped smoking 10 years ago.
For Case three, the NOF and the AACE have no recommendations. The OPOT would suggest starting the patient on a bisphosphonate; a decision supported Dr. Brown. If the patient is hypogonadal, add testosterone and consider calcitonin if he is immobilized with acute pain. Studies indicate beneficial effects of using a bisphosphonate in men with osteoporosis.
Case Four
An 83-year-old Caucasian woman who resides in a nursing home. Her health is poor and although she walks, she has difficulty getting out of a chair. There is no information on her BMD but she has had one fall in the past year.
According to the NOF guidelines, for women who are at least 70 years of age and have multiple risk factors, treatment can be initiated without BMD. Studies show that bisphosphonates, calcium and Vitamin D are beneficial for preventing hip fractures. A study on the effects of supplementation with vitamin D3 (cholecalciferol) and calcium on the frequency of hip fractures and other nonvertebral fractures (n=3270) found that hip fractures were 43% lower, and non-vertebral fractures were 32% lower, in the treatment group when compared to placebo.2 So, in this case it may be beneficial to start calcium and vitamin D therapy and perhaps the use of hip protectors.
Case Five
A 56-year-old woman started HRT four years ago for prevention of heart failure (HF) and cardiovascular disease (CVD). She had an MI 18 months ago and discontinued the HRT for three months. She then started again due to vaginal dryness. She was diagnosed with osteoporosis two years ago (BMD: spine -3.0, hip:-2.5 and had not changed in two years). Total cholesterol 52mmol/L, LDL: 2.8mmol/L, HDL: 1mmol/L and TG 2.8 mmol/L)
Should the patient stay on estrogen or should another agent be added? There is no evidence with estrogen that maintaining BMD is sufficient to reduce hip fracture. She has been on HRT for four years (with the exception of the three-month break) and her BMD has remained constant for two years. The Heart and Estrogen/progestin Replacement Study (HERS) trial examined whether estrogen plus progestin therapy alters the risk for CHD events in postmenopausal women with established coronary disease.3 No significant difference was found between groups on non-fatal and fatal CHD, leading the authors to recommended against starting this treatment for the purpose of secondary prevention of CHD. However, over four years, there was a trend toward fewer CHD events in the treatment group; thus, it could be appropriate for women already receiving this treatment to continue. Another study looked at the effect on quality of life of estrogen plus progestin therapy used as secondary prevention in women with coronary artery disease.4 The effects of hormone therapy depended on the presence of menopausal symptoms; women without flushing had greater declines in physical measures, while women with flushing had improvements in emotional measures of quality of life. For Case five, it is recommended to stop the HRT and initiate bisphosphonate therapy. Use Raplens® or Estring® for the vaginal dryness.
II. Diabetes and the Elderly--How low can you really go?
Treatment of elderly patients with Diabetes Mellitus (DM) is complicated. The aim of this talk was to address two important considerations when treating the elderly patient:
- How low can we go (referring to blood glucose levels)?
- Which medications are optimal for treating the elderly and why?
The prevalence of diabetes increases with age, approaching 20% in Caucasian patients over the age of 70, and in certain ethnic groups, is as high as 50%.5 Many members of the general population have undiagnosed diabetes or have impaired glucose tolerance. Most elderly patients with diabetes are asymptomatic and are diagnosed during a routine visit to the physician's office or after being hospitalized for a complication of diabetes. Most of these patients have Type 2 diabetes and can present with any of the three conditions that are present in most diabetic patients--hypertension, coronary heart disease and hyperlipidemia. However, for the elderly patient, multiple comorbid illnesses, as well as the issue of polypharmacy, may impact the type and extent of treatment.
DM is the sixth most common cause of death among the elderly. Patients with DM have an increased risk of macrovascular and microvascular disease when compared to non-diabetic elderly persons. Longitudinal studies have demonstrated that mortality is strongly correlated with variability in blood glucose and HbA1c, similar to what is seen in the younger diabetic population. DM is a strong indicator of functional decline and has also been correlated with decreased quality of life, increased chronic disease and an increase in utilization of health care resources when compared to non-diabetic elderly. The primary reasons for treating diabetes are improvement of symptoms and avoidance of associated complications (Table 1).
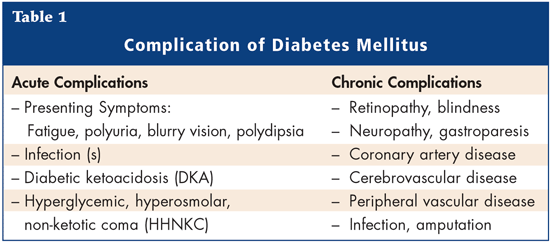
Several studies show that tight control of blood glucose is important for decreasing the long-term complications of DM, and the risk of hypoglycemia in the elderly, which can have serious short-term effects.6-8 The Diabetes Control and Complications Trial (DCCT)6 was conducted to determine whether intensive therapy (with an aim to maintain normal glucose and HbA1c concentrations) could prevent or delay long-term complications in patients with Type 1 DM. The trial showed that during an average treatment period of 6.5 years, the risk of the development or progression of early microvascular complications of diabetes was substantially lower in the intensive-therapy group relative to the conventional-therapy group. Another randomized controlled trial, the United Kingdom Prospective Diabetes Study (UKPDS),7 compared the effects of intensive blood-glucose control with either sulfonylurea or insulin to conventional treatment on the risk of microvascular and macrovascular complications in patients with Type 2 diabetes. Intensive blood-glucose control by either sulfonylureas or insulin substantially decreased the risk of microvascular complications, but not macrovascular disease, in this group of patients. There are some limitations to these trials. The UKPDS used newly-diagnosed patients that were treatment naïve and had less severe disease. Patients with significant comorbid illnesses were excluded, as were elderly patients (over 65 years of age). The DCCT looked at patients with Type 1 DM with a mean age of 27 years.6
There are some specific issues that must be taken into consideration when treating a patient with DM. First, the individual treatment goals must be defined early on. The Canadian Diabetes Association's guidelines for glucose control give a target of 4-7% mmol/L preprandial glucose. Are you trying to achieve symptom control or prevent complications? The extent and impact of comorbidities such as CAD, microvascular complications, functional limitations and disabilities, as well as cerebrovascular and peripheral vascular disease must also be considered. The aforementioned can all affect the ability to achieve tight blood glucose control, an individual's ability to take medication and adherence to a specific regimen. It is also important to assess the risk of and treat hypoglycemia. When reviewing the treatment options, one must also consider the patient's life expectancy and the time frame in which the benefits of treatment will be achieved.
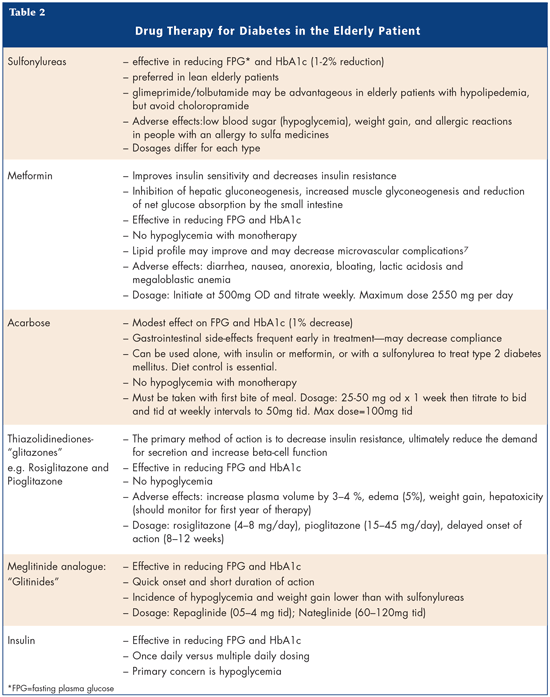
So what are the treatment options for the elderly? A number of drug therapies are available (Table 2). Sulfonylureas or Metformin are the first drugs of choice followed by Glitazones, Glitinide, Acarbose and Insulin.
To summarize, the goal of treating diabetes in the elderly can be similar to that of younger people, to go "low." However, multiple factors and comorbid conditions need to be considered on an individual basis. The goal of treatment should be to maximize benefits and minimize risk.
III. Pharmacological Management of Congestive Heart Failure: Drug Interactions, Comorbid Conditions and New Therapeutic Options
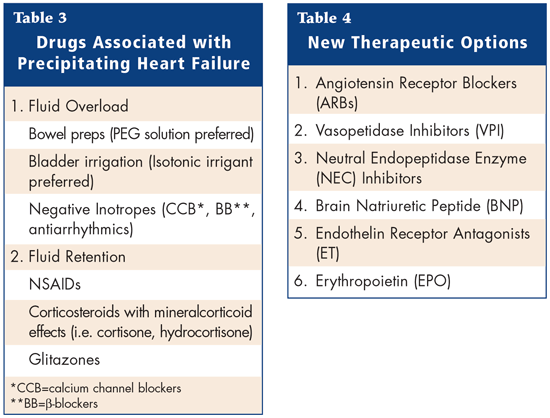
There are numerous drugs that may potentially induce Congestive Heart failure (CHF) in patients with normal ventricular function and/or precipitate heart failure in patients with compensated CHF. Many heart disease patients are elderly and have concomitant diseases requiring multiple medications. It is estimated that medications with contraindications or precautions for use are administered to up to 15% of patients with heart failure. Table 3 gives a summary of the drugs associated with precipitating HF. This talk concentrated on the risk of CHF associated with the use of Non-steroidal anti-inflammatory drugs (NSAIDs)--including COX-2 inhibitors.
NSAIDs are the most widely used therapeutic agents in the elderly, generally for the management of osteoarthritis and rheumatoid arthritis. NSAIDs reversibly inhibit the cyclooxygenase (COX) enzyme and prevent the biosynthesis of prostaglandins. In patients with HF, prostaglandin synthesis is an important compensatory mechanism for the maintenance of cardiovascular and renal homeostasis. By blocking the synthesis of prostaglandins, NSAIDs may interfere with renal homeostasis inducing or exacerbating HF. NSAIDs also interact with Angiotensin Converting Enzyme (ACE) inhibitors. Both angiotensin II (efferent arteriole vasoconstrictor) and prostaglandins (afferent arteriole vasodilator) play a role in preserving renal function. ASA has been reported to reduce efficacy of ACE inhibitors, so it is advisable to limit ASA dose to less than 100 mg per day in HF patients receiving an ACE inhibitor. There is also an increased risk of hyperkalemia with concomitant use of NSAIDs and ACE inhibitors.
The COX-1 enzyme is present in most tissues, and functions as a housekeeping enzyme, increasing prostaglandins that mediate homeostatic functions such as platelet activation, renal perfusion and maintainence of normal gastric mucosa. Blocking COX-1 can result in serious gastrointestinal adverse effects. COX-2 is induced by inflammation and increases prostaglandins that mediate pathologic effects such as local inflammation, pain and fever. COX-2 also increases prostaglandins that mediate renal homeostasis. The development of newer COX-2 selective drugs has allowed for relief of pain and inflammation without the adverse effects associated with COX-1 blockade. However, by decreasing vasodilatory and antiaggregatory prostacyclin production, COX-2 antagonists may lead to increased prothrombotic activity. A meta-analysis of trials (including the Vioxx Gastrointestinal Outcomes Research Study (VIGOR; 8076 patients)) and the Celecoxib Long-term Arthritis Safety Study (CLASS; 8059 patients) found that the annualized myocardial infarction rates for patients taking COX-2 inhibitors in both VIGOR and CLASS were significantly higher than those in the placebo group.9 The available data raise a cautionary flag regarding the risk of cardiovascular events with COX-2 inhibitors and further prospective trial evaluation may characterize and determine the magnitude of the risk.9
There are a number of risk factors for NSAID renal toxicity. These include pre-existing renal disease, renal hyperfusion and concomitant drug therapy (diuretics and antihypertensives). NSAIDs have been associated with hospitalization for congestive heart failure.10,11 In patients with pre-existing HF, use of NSAIDs is associated with a substantially increased risk of a relapse. It has also been reported that the risks are greatest for NSAIDs with a long half-life (naproxen, piroxicam).
Uncontrolled hypertension is a precipitating factor for HF in 44% of patients.11 NSAIDs cause sodium and water retention (fluid retention 05-1L; weight gain 1-2 kg). NSAIDs also inhibit synthesis of prostacylin (PGI2) and increase peripheral resistance. Thus, they can elevate blood pressure, especially in those patients who have pre-existing hypertension. Studies also report that NSAIDs blunt the therapeutic effects of antihypertensive medications.11
Approximately 10% of elderly patients use NSAIDs and diuretics at least once a year. NSAIDs can alter the therapeutic effects of diuretics, especially in patients with depleted sodium levels. There is also an increase risk of hyperkalemia with concomitant use of NSAIDs and spironolactone.
Therefore, NSAIDs (including COX-2 inhibitors) must be used with caution in patients with HF and wherever possible, alternative pharmacologic agents should be used. If an NSAID is indicated, the patient should be informed of the potential risks and need for regular monitoring of serum creatinine, body weight and signs and symptoms of HF. There are a number of new therapeutic options. Table 4 summarizes the new options available.
IV. New Evidence and Guidelines in Osteoarthritis (OA)
Using a "typical" case, Dr. Kennie identified management issues for the use of pharmacologic alternatives in OA in the elderly. She outlined the recent changes in guidelines for the management of OA and the supporting evidence, and discussed common management issues for specific pharmacalogic alternatives in the elderly.
Case: OTIS
A 67-year-old retired stockbroker who has had osteoarthritis in his knee for the last three years. Recently, his knee pain has worsened and he has noticed stiffness and soreness for 30 minutes when he wakes up in the morning or after sitting for long periods. He has some pain at rest and has noticed a clicking sound when he walks. Otis probably has mild OA. He has been taking acetaminophen 500 mg, 1-2 tablets three times a day on most days and feels that it is somewhat useful.
Osteoarthritis (OA) is a slowly progressive disorder of the joints; very little inflammation is involved in the early stages, although some believe that there is inflammation at the molecular level. OA most commonly involves the hands, feet, spine and weight-bearing joints such as the knees and hips. There are two main factors that lead to joint failure. The first is progressive breakdown of articular cartilage that lines joint surfaces, which is associated with pain and disability. The second is the formation of dense, smooth surface bone at the base of the cartilage lesion and the formation of osteophytes. The goals of therapy for OA should be to control pain, maintain joint function, reduce disability and improve health-related quality of life. The question is when to treat and with what? This depends on the severity of the OA pain. Guidelines on pharmacologic therapy suggest the following:
- The use of Acetaminophen for mild-to-moderate OA (where pain occurs occasionally). Acetaminophen has an analgesic role, but is not an anti-inflammatory agent. Acetaminophen has demonstrated similar efficacy to NSAIDs for relief of mild-to-moderate OA,12 and is a safer, lower cost alternative. Acetaminophen has the added benefit of being used on either an as needed or regular basis. However, it should be used with caution in patients consuming excessive alcohol or in patients with liver damage. For adequate OA control, a dose of 1g qid is required.
- For moderate-to-severe OA pain (pain occurring more frequently, with some disability), NSAIDs should be considered when symptoms are not adequately controlled by acetaminophen. This higher efficacy of NSAIDs may relate to the fact that inflammation is occurring in OA earlier than was previously believed. Patients tend to prefer NSAIDs, which are superior to acetaminophen for pain at rest and pain on motion.2 There are several types of NSAIDs (salicylates, traditional, and COX-2 selective NSAIDs) and they are generally equivalent in efficacy for OA pain at comparable doses. The agents with a longer half-life may provide better pain control (e.g. meloxicam).
- For single joint involvement, topical analgesics and intra-articular injections can be considered.
Certain factors must be taken into consideration when prescribing NSAIDs for elderly patients, including the risk of GI toxicity, the risk of renal toxicity, congestive heart failure or high blood pressure. Inhibition of COX-1 can lead to gastrointestinal mucosal injury. In patients over 65 years, research shows that 20-30% of all hospitalizations and deaths due to peptic ulcer disease were attributable to NSAIDs.14 It has also been reported that 20-25% of patients experience dyspepsia during therapy, the severity of which is unrelated to the severity of the mucosal injury. The risk factors for GI toxicity include: previous peptic ulcer disease, age over 65 years, concomitant use of warfarin or corticosteroids, comorbid illness, chronic alcoholism and the use of multiple medications. For high-risk patients, it is recommended to use COX-2 selective inhibitors or a traditional NSAID plus a gastroprotective agent (e.g. PPI).
Other agents may also be used for OA. Narcotic analegesics13,15 relieve pain but lack anti-inflammatory properties. They are generally used to provide short-term analgesia when pain has become very severe. They may also be considered in patients who do not respond or have contraindications to acetaminophen or NSAIDs, or are not candidates for surgery for chronic pain management. Patients may experience adverse effects such as nausea, constipation, dizziness or drowsiness.
Recently, there has been new evidence on the use of glucosamine sulfate.16,17,18 Glucosamine is believed to stimulate the production of cartilage and prevent its destruction by inflammatory mediators and enzymes. Glucosamine can be beneficial in reducing pain (mild-to-moderate) in OA patients. One study suggested that it may slow the progression of OA in the knee;18 however, the agent used in the study was an oral form of a specific glucosamine crystalline product made in Belgium. The usual dose is 1500mg/day (od or tid) and the full effects are not seen for 4-6 weeks. A common side effect is GI upset. Glucosamine should be used with caution in patients with diabetes due to potential inteference with glucose control.
References
- Lindsay R, Cosman F, Lobo RA, et al. Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomized, controlled clinical trial. J Clin Endocrinol Metab. 1999;84:3076-81.
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992; 327:1637-42.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998; 280:605-13.
- Hlatky MA, Boothroyd D, Vittinghoff E, et al. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial. JAMA. 2002; 287:591-7.
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The third national health and nutrition examination survey, 1988-1994. Diabetes Care 1998;21:518-24.
- Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 2000;342:381-9.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-53.
- Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995 28:103-17.
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954-9.
- Feenstra J, Heerdink ER, Grobbee DE, et al.Association of Nonsteroidal Anti-inflammatory Drugs With First Occurrence of Heart Failure and With Relapsing Heart Failure.The Rotterdam Study Arch intern Med 2002;162-265-70.
- Page J, Henry D. Consumption of NSAIDs and the Development of Congestive Heart Failure in Elderly Patients : An Underrecognized Public Health Problem. Arch intern Med 2000;160:777-84.
- Bradley JD, Brandt KD, Katz BP, et al. Comparison of an antiinflammatory dose of ibuprofen, an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N Engl J Med. 1991;325:87-91.
- Tannenbaum H, Peloso PM, Russell AS, Marlow B. An evidence-based approach to prescribing NSAIDs in the treatment of osteoarthritis and rheumatoid arthritis: The Second Canadian Consensus Conference. Can J Clin Pharmacol. 2000;7 Suppl A:4A-16A. Review.
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxiciy of nonsteroidal antiinflammatory drugs. N Engl J Med 1999;340:1888-99.
- Canadian Pain Society. Use of opioid analgesics for the treatment of chronic noncancer pain. Pain Res Mang 1998;3:197-208.
- da Camara CC, Dowless GV. Glucosamine sulfate for osteoarthritis. Ann Pharmacother. 1998 May;32:580-7. Review.
- McAlindon TE, LaValley MP, Gulin JP et al. Glucosamine and chrondroitin for treatment of osteoarthritis: A sytematic quality assesement and meta-analysis. JAMA. 2000;283:1469-75.
- Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251-6.



