Sarcopénie et vieillissement musculaire
Conférencier : Patrick Dehail, M.D., Ph. D., service de Médecine Physique et Réadaptation & EA 4136, CHU de Bordeaux, Université de Bordeaux 2; Bordeaux, France.
Le Dr Patrick Dehail s'est intéressé aux mécanismes, à l’impact fonctionnel et aux approches thérapeutiques de la sarcopénie et du vieillissement musculaire.
Définition de la sarcopénie
La sarcopénie est une perte progressive de la masse musculaire associée au vieillissement. Sa prévalence est élevée, entre 10 et 24 % de la population âgée de 65 à 70 ans et jusqu'à plus de 30 % après 80 ans.
La sarcopénie se définit par un index de masse musculaire (IMM) (masse musculaire appendiculaire [kg]/taille2 [m2]) inférieur à au moins deux écarts-type par rapport à celui d’une population de référence plus jeune1. Il serait très utile de disposer d’un seuil qui tienne compte de la performance musculaire et qui décrive la perte de masse musculaire associées aux conséquences fonctionnelles.
Chez la personne âgée, le déclin de la masse musculaire est associé à une augmentation de la masse grasse. Il est important de considérer les deux phénomènes, puisque les conséquences de la sarcopénie seront différentes en fonction de la masse grasse.
Modifications du tissu musculaire liées au vieillissement et mécanismes associés
La sarcopénie est associée à des modifications du tissu musculaire, notamment une réduction du nombre de fibres de type II et une atrophie de ces fibres, les fibres de type I étant relativement épargnées (Figure 1). Au niveau moléculaire, le vieillissement est associé à une diminution de l’expression des isoformes des chaînes lourdes de myosine (MHC) de type IIa et IIx, sans grande modification de l’expression de la MHC de type I. On observe également une augmentation du nombre de fibres hybrides, qui vont co-exprimer différents types d’isoformes de MHC.
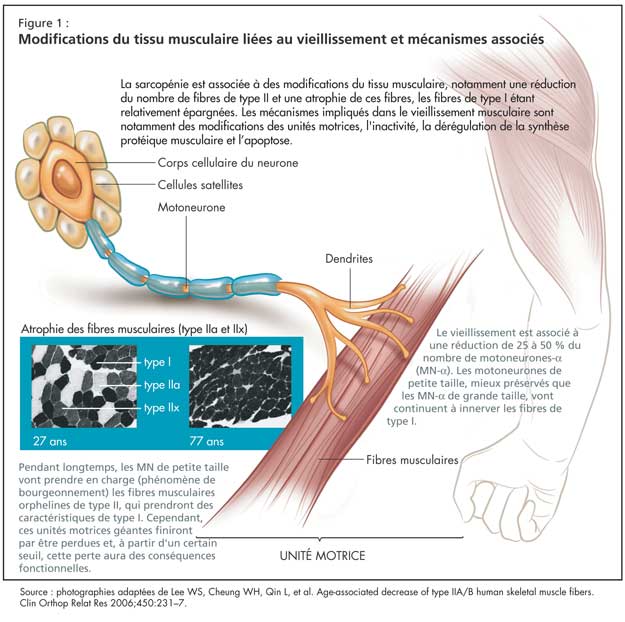
Les mécanismes impliqués dans le vieillissement musculaire sont notamment des modifications des unités motrices, l'inactivité, la dérégulation de la synthèse protéique musculaire et l’apoptose.
Le vieillissement est associé à une réduction de 25 à 50 % du nombre de motoneurones-a (MN-a). Les MN de petite taille, mieux préservés que les MN de grande taille, vont continuer à innerver les fibres de type I. La perte des MN-a de grande taille est compensée pendant longtemps par un phénomène de bourgeonnement, où les MN de petite taille vont prendre en charge les fibres musculaires orphelines de type II, qui prendront des caractéristiques de type I. Ce phénomène de bourgeonnement a des limites, cependant, et ces nouvelles unités motrices géantes finiront par être perdues. À partir d’un certain seuil, cette perte aura des conséquences fonctionnelles.
On considère l’inactivité comme un facteur étiologique du phénomène sarcopénique. Cependant, on ne sait pas dans quelles proportions l’inactivité est la conséquence des modifications neuromusculaires (phénomène adaptatif) ou contribue à ces changements.
Le Dr Dehail a expliqué que la dérégulation de la synthèse des protéines musculaires est un élément central du phénomène sarcopénique. La séquestration splanchnique détourne les acides aminés vers le foie ou l'intestin. D’autre part, l'insulino-résistance, dont la prévalence augmente avec l'âge, joue un rôle défavorable en augmentant la protéolyse des protéines musculaires. La diminution du taux des hormones anaboliques (testostérone, axe GH-IGF1, DHEA) contribue également à ce trouble. Enfin, l'augmentation du taux des cytokines pro-inflammatoires (notamment l'Il6 et le TNF-a) chez la personne âgée stimule le processus protéolytique. La diminution du taux de MGF (facteur qui stimule le pool des cellules satellites), l’augmentation du taux de myostatine (inhibiteur de la croissance musculaire) et l’apoptose contribuent aussi à la sarcopénie.
Le vieillissement est également associé à des troubles de la microcirculation qui affectent le tissu musculaire.
Les personnes âgées présentent fréquemment une malnutrition, une anorexie et une diminution du taux de vitamine D et du nombre de récepteurs VDR à la vitamine D. La prévalence de la carence en vitamine D dépasse les 90 % chez les personnes âgées hospitalisées. La vitamine D agit sur les capacités fonctionnelles du tissu musculaire et sur la synthèse protéique, et il existe une corrélation entre le taux de 25-hydroxy vitamine D (25-OHD) et la perte de force musculaire. Visser et ses collègues ont montré que les personnes qui ont un taux de 25-OHD < 25 nmol/l étaient plus susceptibles de perdre leur force de préhension à trois ans. Une diminution du taux circulant de 25-OHD a également été corrélée à une diminution du temps de marche ou du temps de réalisation d’une épreuve de lever de chaise2.
Impact fonctionnel du vieillissement musculaire
Les modifications du tissu musculaire vont retentir sur la performance et la force musculaire. Cette perte de force musculaire commence tôt, mais va rester insignifiante jusqu'à 50-60 ans. C’est surtout la force musculaire isocinétique, concentrique et des membres inférieurs qui est initiatement touchée. De plus, la perte de force musculaire est asymétrique au niveau des groupes musculaires antagonistes.
Le seuil de tolérance en matière de perte fonctionnelle varie en fonction des individus et de la tâche fonctionnelle, mais des auteurs ont proposé des seuils cliniques au-dessous desquels la majorité des sujets connaîtront des problèmes. Par exemple, Janssen et ses collègues ont trouvé qu’un IMM inférieur à 5,75 chez les femmes et 8,5 chez les hommes était corrélé à un niveau plus élevé d’incapacité fonctionnelle3. Ploutz-Snyder et ses collègues estiment qu’un rapport « force isométrique des quadriceps/poids corporel » < 3 Nm/kg est associé à une dégradation des capacités fonctionnelles, notamment celles en rapport avec la locomotion (marche, montée de marches d’escalier, transfert assis-debout)4. Lauretani et ses collègues ont proposé une méthode encore plus simple, montrant qu’une force de préhension inférieure à 30 kg pour les hommes ou 20 kg pour les femmes permet d’identifier les personnes âgées ayant une vitesse de marche ou des capacités locomotrices moindres5.
Sur le plan fonctionnel, il est important de considérer la perte de puissance musculaire, car elle est impliquée dans beaucoup d'activités basiques importantes pour la personne âgée, comme le transfert assis-debout, la montée de marches d'escalier ou la marche. La puissance musculaire est extrêmement utile en situation de déséquilibre (chute), car elle permet un réajustement postural. Elle permet également aux personnes âgées de conserver leur indépendance.
Avec l'âge, la qualité musculaire (mesure de force par unité de masse musculaire) se dégrade également. Une détérioration de la conduction de la voie corticospinale et une augmentation des co-contractions entre muscle agoniste et antagoniste parasitent le mouvement volontaire. On observe également des modifications des propriétés rhéologiques du muscle squelettique, avec une prolongation du temps nécessaire pour obtenir une contraction musculaire et une augmentation du temps de demi-relaxation. Une diminution de la raideur tendineuse va entraver la transmission de la force musculaire au segment articulaire.
Enfin, le Dr Dehail a parlé de la notion importante de force soutenue (capacité à maintenir un niveau de contraction maximal lors d’un effort soutenu). Le Dr Dehail et ses collègues ont comparé le coefficient d'endurance isocinétique (rapport de force entre les trois dernières contractions musculaires concentriques par rapport aux trois premières) et l'évolution de la perte de force musculaire chez des personnes jeunes et âgées6. Les personnes les plus âgées et les plus fragiles (moyenne d'âge : 85 ans) ne montraient pas de perte de force, et leur coefficient d'endurance restait proche de 1. Chez des sujets un peu moins âgés (moyenne d’âge : 75 ans), le coefficient d'endurance passait à 0,92, par rapport à 0,85 chez des sujets jeunes (étudiants). En fait, lors d'un effort soutenu, les personnes plus jeunes vont d’abord utiliser les fibres de type II, puis les fibres de type I. Les personnes âgées fragiles et hospita-lisées vont mettre en jeu directement les fibres de type I, ce qui explique que leur coefficient d'endurance reste proche de 1.
Approches thérapeutiques
Renforcement musculaire
Le renforcement musculaire reste essentiel pour lutter contre la sarcopénie : c’est la méthode qui s’est montrée le plus efficace. Tous les types de renforcement musculaire sont appropriés, mais il faut adapter le mode d'exercice au patient. Les protocoles varient, mais en moyenne il faut compter au moins trois séances par semaine, pendant 12 semaines (temps nécessaire pour obtenir le gain de force maximale).
Durant les trois premières semaines, les performances s’améliorent sans adaptation au niveau nerveux ou musculaire. De la 3e à la 6e semaine, le gain de force est le plus important, principalement en raison du mécanisme d'adaptation des unités motrices (meilleur recrutement, augmentation de la fréquence de décharge, meilleure synchronisation) et à une diminution des phénomènes de co-activation musculaire. De la 6e à la 12e semaine, ce sont les mécanismes d'adaptation musculaire qui prédominent (légère augmentation de masse musculaire et hypertrophie modérée des fibres). Au-delà de 12 semaines de renforcement musculaire, la personne âgée ne va plus gagner en force musculaire, mais va maintenir la puissance musculaire tant qu’elle continue les exercices au même rythme.
Le Dr Dehail a cité une étude de Yarasheski et ses collègues, qui ont montré que, au terme de deux semaines de renforcement musculaire, la synthèse de protéines musculaires était équivalente chez les sujets jeunes (23-32 ans) et les sujets âgés (78-84 ans)7.
Dans une revue systématique, Latham et ses collègues ont montré que les sujets âgés qui suivent un programme de renforcement musculaire améliorent considérablement leur force musculaire (augmentation de 20 à 200 % de la 1 RM [répétition maximale] selon les études)8.
Le renforcement musculaire améliore la force et la puissance musculaires, améliore légèrement la vitesse de marche et le temps de transfert assis-debout, et réduit le risque de chute. Néanmoins, on n’en connaît pas bien les conséquences sur les AVQ ou sur la qualité de vie. L’association d’une supplémentation nutritionnelle est importante pour optimiser les résultats chez les personnes âgées malnutries.
Médicaments
Selon le Dr Dehail, la testostérone et l'hormone de croissance (GH) n’améliorent les performances musculaires que pour les sujets hypogonadiques ou ayant une déficience en GH. La DHEA ne montre aucun bienfait en terme de performance musculaire. La vitamine D diminue le risque de chute, mais cet effet bénéfique ne semble pas être directement associé à une amélioration de la force ou de la puissance musculaire.
Les inhibiteurs de l’ECA pourraient s’avérer bénéfiques. Une étude d'observation a montré des résultats positifs sur la puissance et la masse musculaires. Des essais comparatifs sont nécessaires pour confirmer ces résultats.
D'autres molécules sont à l'étude, comme les SARM (des modulateurs des récepteurs aux androgènes sélectifs), les inhibiteurs de la myostatine ou certains acides aminés (leucine).
Conclusion
La sarcopénie est corrélée à une augmentation de l’incapacité fonctionnelle dans les AVQ, à une augmentation du risque de chute, à un syndrome de fragilité et à un état de dépendance. Elle est également associée à un taux plus élevé de mortalité, notamment chez les personnes âgées hospitalisées, en raison d’une augmentation des infections nosocomiales.
Enfin, la sarcopénie est associée à une augmentation très nette du coût des soins de santé (surcoût d’environ 900 $ par an et par patient âgé sarcopénique aux É.-U.).
Bibliographie
-
Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147:755-63.
-
Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003; 88:5766-72.
-
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413-21.
-
Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, et al. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci 2002;57:B144-52.
-
Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851-60.
-
Muller F, Dehail P, Bestaven E, Petit J, Joseph PA, Barat M, et al. Maximal and sustained isokinetic lower-limb muscle strength in hospitalized older people. Muscle Nerve 2007;35:739-44.
-
Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci 2003;58:M918-22.
-
Latham NK, Bennett DA, Stretton CM, et al. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci 2004;59:48-61.