
Advances in Influenza Vaccination
New Technology in Influenza Vaccination
Speaker: David P. Greenberg, MD, Senior Director, Scientific and Medical Affairs, US, Sanofi Pasteur; Adjunct Associate Professor of Pediatrics, University of Pittsburgh School of Medicine; Pediatric Infectious Diseases, Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA.
Dr. David Greenberg’s discussion focused on novel technologies that could improve the immunogenicity achieved with influenza vaccines as well as increase vaccination rates among both older and younger adults.
Dr. Greenberg initially focused on results of studies with a high-dose intramuscular vaccine tested on older adults. Older adults’ declining humoral and cellular immunity, due to immunosenescence, increases their susceptibility to infection and decreases their immunologic responses to vaccines. As a result, older adults’ response to vaccination may be poor, yielding fewer protective antibodies. Higher-dose vaccines are being pursued to overcome this limitation.
Dr. Greenberg detailed a Phase 3 clinical trial of a high-dose influenza vaccine (60µg hemagglutinin [HA]/strain [H1N1, H3N2, and B]) that found value to the approach.1 This randomized multicentre trial of 3,876 individuals, all ≥65 years of age and medically stable, compared high-dose vaccine versus standard-dose vaccine (Fluzone®, sanofi pasteur; 15µg HA/strain). The high-dose trivalent, inactivated influenza vaccine offered a fourfold higher antigen content compared with standard dose vaccine. Researchers reported significantly higher seroconversion and seroprotection rates and significantly higher hemagglutination inhibition (HAI) geometric mean antibody titres (GMTs) 28 days after vaccination among subjects who received high-dose vaccine compared with those who received standard-dose vaccine. Using strict U.S. Food and Drug Administration criteria, the high-dose vaccine demonstrated statistically superior immunogenicity compared with standard-dose vaccine (immunologic superiority for both A strains [H1N1 and H3N2] and noninferiority for the B strain). Local injection site reactions occurred more frequently in individuals who received the high-dose vaccine, but the reactions were generally mild to moderate.
Influenza-associated morbidity and mortality remains substantial among older adults, Dr. Greenberg emphasized, and the improved immunogenicity response elicited by the high-dose vaccine is likely to provide improved protective benefits for this population.
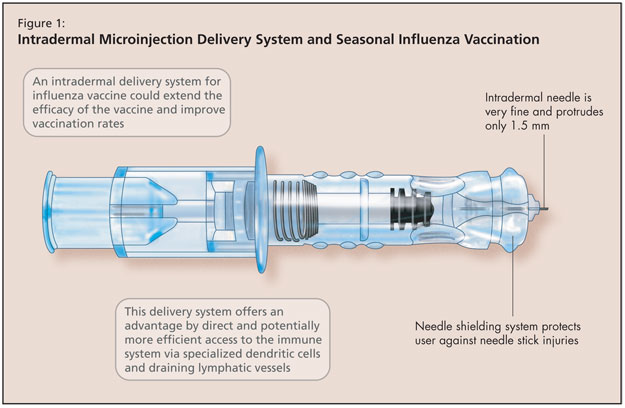
The next development in immunization research Dr. Greenberg discussed concerned seasonal influenza vaccination by intradermal microinjection (Figure 1). This is another approach to address the reduced immunogenicity of influenza vaccines among older adults that results from immunosenescence. Additionally, in healthy younger adults, vaccine uptake remains low. An intradermal delivery system offers an alternative that may improve vaccination rates and extend protection to people who might not otherwise receive annual influenza vaccination.
The physiologic principle of intradermal vaccination takes advantage of dendritic cells, which are the antigen presenting cells in the dermal layer. The dermal layer is also rich in lymphatic and blood supply, making it a robust arm of the immune system.
Dr. Greenberg reviewed results of two relevant Phase 2 clinical studies of intradermal vaccines.
The first was a multicentre, randomized study of 1,107 volunteers ≥60 years of age.2 Participants received intradermal trivalent inactivated influenza vaccine containing 15 or 21mg of HA per strain or intramuscular control vaccine (Vaxigrip®, sanofi pasteur, 15 mg HA/strain). The primary endpoints of the study were the strain-specific HAI GMTs 21 days after vaccination. The authors of the study reported that, for each strain, the GMTs noted in association with each intradermal vaccine were superior to those noted with the intramuscular control.
The second was a Phase 2, multicentre, randomized open-label study of 978 healthy adults under age 60, which evaluated the immunogenicity and safety of intradermal trivalent inactivated influenza vaccine.3 Participants were randomized to either 0.1 ml intradermal vaccine with reduced antigen (9mg HA per strain) or conventional 0.5 ml intramuscular vaccine (Vaxigrip vaccine). Intradermal vaccination induced noninferior humoral immune responses against all three strains compared with intramuscular vaccine. Dr. Greenberg noted that conventional intramuscular vaccination induces strong immune responses in younger adults, but immunization rates need to be improved in this population. One means of achieving higher rates is through the alternative of this intradermal microinjection delivery system.
The intradermal needle used in these studies consisted of a very fine 30-gauge needle that protrudes only 1.5 milimetres (BD microinjection system [Becton Dickinson]). In addition to the advantages offered by this direct and potentially more efficient access to the immune system (via specialized dendritic cells and draining lymphatic vessels in the dermis), a needle shielding system protects the user against needle stick injuries.
Dr. Greenberg concluded that the studies offered sound evidence that intradermal vaccination can be used to elicit higher immune responses against seasonal influenza among older adults at a dosage of 15µg HA/strain, and is a promising alternative to intramuscular vaccination for adults <60 years of age, at a dosage of 9µg HA/strain.
The safety profile of intradermal influenza vaccination is comparable with conventional intramuscular vaccination but with higher rates of minor injection site reactions.
The final approach Dr. Greenberg discussed was adjuvanted vaccines. He provided details of a Phase 1 clinical study of adjuvanted low-dose H5N1 (avian strain) vaccine conducted among participants age 18-40 years.4 Groups of 50 participants received 2 doses, 21 days apart, of influenza A/Vietnam/1194/2004 NIBRG-14 (H5N1) vaccine containing 1.9, 3.8, 7.5, or 15µg of HA mixed with an oil-in-water emulsion adjuvant or 7.5µg of HA without adjuvant. Homologous HAI and microneutralization titres were determined after each vaccination. Cross-reactivity against A/Indonesia/05/2005 RG2 was tested after the second vaccination. Dr. Greenberg described robust seroconversion rates with adjuvanted vaccine (72–89%) compared with unadjuvanted vaccine (34%). The adjuvanted H5N1 vaccine was well-tolerated, and adequate immune responses were observed with as little as 1.9µg HA. Further, antibodies induced by adjuvanted vaccine were crossreactive to another strain of avian influenza, clade 2 Indonesia/5/05 RG2 strain, not observed with unadjuvated vaccine. All strengths of the adjuvanted vaccine met European Committee for Medicinal Products for Human Use immunogenicity criteria.
The fundamental benefit of adding an adjuvant to H5N1 vaccine, Dr. Greenberg emphasized, is that it is dose-sparing. An emulsion-adjuvanted pandemic influenza vaccine could have a major, positive effect on pandemic vaccination strategies due to limited vaccine stockpiles and limited worldwide manufacturing capacity.
In closing, Dr. Greenberg highlighted that the morbidity and mortality of influenza remains substantial across all age groups, particularly among older individuals. The novel vaccination strategies he discussed could help improve immunologic responses among older persons, overcome suboptimal immunization rates among high-risk and healthy younger adults, and better prepare health professionals to meet an influenza pandemic.
References:
- Falsey AR, Treanor JJ, Tornieporth N, et al. Randomized, double-blind controlled Phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009;200:172–80.
- Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008;198:650–8.
- Leroux-Roels I, Vets E, Freese R, et al. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine 2008;26:6614–9.
- Levie K, Leroux-Roels I, Hoppenbrouwers K, et al. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis 2008;198:642–9.
Sponsored by an unrestricted educational grant from sanofi pasteur.


